Our garden soil
One thing a gardener is going to be curious about is what’s in their soil, particularly the urban gardener. I took soil samples from my plot and sent them off to the Cornell Nutrient Analysis Lab for testing where they performed their #2020 test (Soil Acid Package or Soil Total Elemental Analysis). This test measures the levels of some acid-soluble elements in the soil, including various metals. Here’s what they found, where the results are expressed in mg/Kg. These values are equivalent to parts per million (ppm).
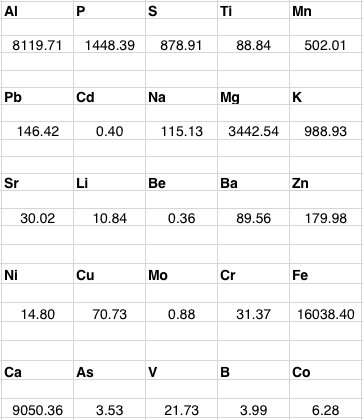
Amongst the elements listed there you have lead (Pb), arsenic (As), and cadmium (Cd), all potentially toxic. Are our levels high? Here’s a table that the lab supplied that shows some recommended maximum levels (our soil type is silt to clay):
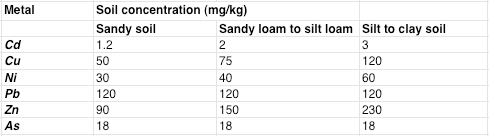
Bottom line, we’re under the recommended maximum levels for arsenic, cadmium, copper, nickel, zinc, and arsenic, but slightly above what Cornell would like to see for lead (120 recommended, 146.42 actual). Is this a problem? You’ll find different opinions on this online but here’s a quote from Lead in the Home Garden and Urban Soil Environment (University of Minnesota):
Since plants do not take up large quantities of soil lead, the lead levels in soil considered safe for plants will be much higher than soil lead levels where eating of soil is a concern. Generally, it has been considered safe to use garden produce grown in soils with total lead levels less than 300 ppm. The risk of lead poisoning through the food chain increases as the soil lead level rises above this concentration. Even at soil levels above 300 ppm, most of the risk is from lead contaminated soil or dust deposits on the plants rather than from uptake of lead by the plant.
Wash or peel your produce, never eat the soil itself, and there’s no risk from this level of soil lead, that’s my interpretation.
If you do more research on this remember these are the total or acid-soluble levels. Many of the elements in the soil, like iron (Fe) and aluminum (Al), are present in multiple forms or salts. Most of these forms are insoluble when the soil pH is neutral, 6 to 8, and these insoluble forms are not taken up by the plants and do not affect the plants (see Behaviour of Metals in Soil for some science on this).
See More on our garden soil for the test results on our soil’s fertility.
Brian O